Workshop
Summarising data in with several variables and the role of variables in analysis
Introduction
Session overview
In this workshop you will learn to summarise and plot datasets with more than one variable and how to write figures to files. You will also get more practice with working directories, importing data, formatting figures and the pipe.
Philosophy
Workshops are not a test. It is expected that you often don’t know how to start , make a lot of mistakes and need help. It is expected that you are familiar with independent study content before the workshop. However, you need not remember or understand every detail as the workshop should build and consolidate your understanding. Tips
- don’t worry about making mistakes
- don’t let what you can not do interfere with what you can do
- discussing code with your neighbours will help
- look things up in the independent study material
- look things up in your own code from earlier workshops
- there are no stupid questions
These four symbols are used at the beginning of each instruction so you know where to carry out the instruction.
 Something you need to do on your computer. It may be opening programs or documents or locating a file.
Something you need to do on your computer. It may be opening programs or documents or locating a file.
 Something you should do in RStudio. It will often be typing a command or using the menus but might also be creating folders, locating or moving files.
Something you should do in RStudio. It will often be typing a command or using the menus but might also be creating folders, locating or moving files.
 Something you should do in your browser on the internet. It may be searching for information, going to the VLE or downloading a file.
Something you should do in your browser on the internet. It may be searching for information, going to the VLE or downloading a file.
 A question for you to think about and answer. Record your answers in your script for future reference.
A question for you to think about and answer. Record your answers in your script for future reference.
Getting started
 Start RStudio from the Start menu.
Start RStudio from the Start menu.
 Make an RStudio project for this workshop by clicking on the drop-down menu on top right where it says
Make an RStudio project for this workshop by clicking on the drop-down menu on top right where it says Project: (None) and choosing New Project, then New Directory, then New Project. Navigate to the data-analysis-in-r-1 folder and name the RStudio Project ‘week-9’.
 Make a new folder called
Make a new folder called data-raw. You can do this on the Files Pane by clicking New Folder and typing into the box that appears.
 Make a new script then save it with a name like
Make a new script then save it with a name like summarise-plot-data-with-several-vars.R to carry out the rest of the work.
 Add a comment to the script:
Add a comment to the script: # Summarising data in with several variables and the role of variables in analysis
 Add code to load the
Add code to load the tidyverse package (Wickham et al. 2019)
Exercises
The Independent Study to prepare for workshop explained how to summarise and plot a dataset containing one continuous response and one nominal explanatory variable with two groups.
Here you are going to learn how to summarise the following types of data set:
- one continuous response and one nominal explanatory variable with three groups: myoglobin concentration in seal species
- one continuous response and one nominal explanatory variable with two groups but the supplied data first need converting to tidy format: interorbital width in pigeons from two populations
- two continuous variables: ulna length and height in humans
You should start to notice how similar the code is for all of these and also where they differ.
Data already tidy: Myoglobin in seal muscle
The myoglobin concentration of skeletal muscle of three species of seal in grams per kilogram of muscle was determined and the data are given in seal.csv. Each row represents an individual seal. The first column gives the myoglobin concentration and the second column indicates species.
Import
 Save seal.csv to your
Save seal.csv to your data-raw folder.
In the next step you will import the daya and I suggest you look up data import from last week. There are two ways you could do that.
Open your own script from last week. You do not need to open the
week-8RStudio Project itself - you can open the script you used by navigating to through the File pane. After you have found it and clicked on it to open, I suggest showing your working directory in your File Pane again. You can do that by clicking on the little arrow at the end of the path printed on the Console window.Go the the workshop page from last week.
 Read the data into a dataframe called
Read the data into a dataframe called seal.
 What types of variables do you have in the
What types of variables do you have in the seal dataframe? What role would you expect them to play in analysis?
A key point here is that the fundamental structure of:
one continuous response and one nominal explanatory variable with two groups (adipocytes), and
one continuous response and one nominal explanatory variable with three groups (seals)
is the same! The only thing that differs is the number of groups (the number of values in the nominal variable). This means the code for summarising and plotting is identical except for the variable names!
When two datasets have the same number of columns and the response variable and the explanatory variables have the same data types then the code you need is the same.
Summarise
Summarising the data for each species is the next sensible step. The most useful summary statistics for a continuous variable like myoglobin are:
- mean
- standard deviation
- sample size
- standard error
We can get those for the whole myoglobin column, like we did for the FSA column in the cells dataset last week. However, it is likely to be more useful to summarise myoglobin separately for each seal species. We can achieve this using group_by() before summarise().
 Create a data frame called
Create a data frame called seal_summary1 that contains the means, standard deviations, sample sizes and standard errors for each of the seal species:
You should get the following numbers:
| species | mean | std | n | se |
|---|---|---|---|---|
| Bladdernose Seal | 42.31600 | 8.020634 | 30 | 1.464361 |
| Harbour Seal | 49.01033 | 8.252004 | 30 | 1.506603 |
| Weddell Seal | 44.66033 | 7.849816 | 30 | 1.433174 |
Visualise
Most commonly, we put the explanatory variable on the x axis and the response variable on the y axis. A continuous response, particularly one that follows the normal distribution, is best summarised with the mean and the standard error. In my opinion, you should also show all the raw data points wherever possible.
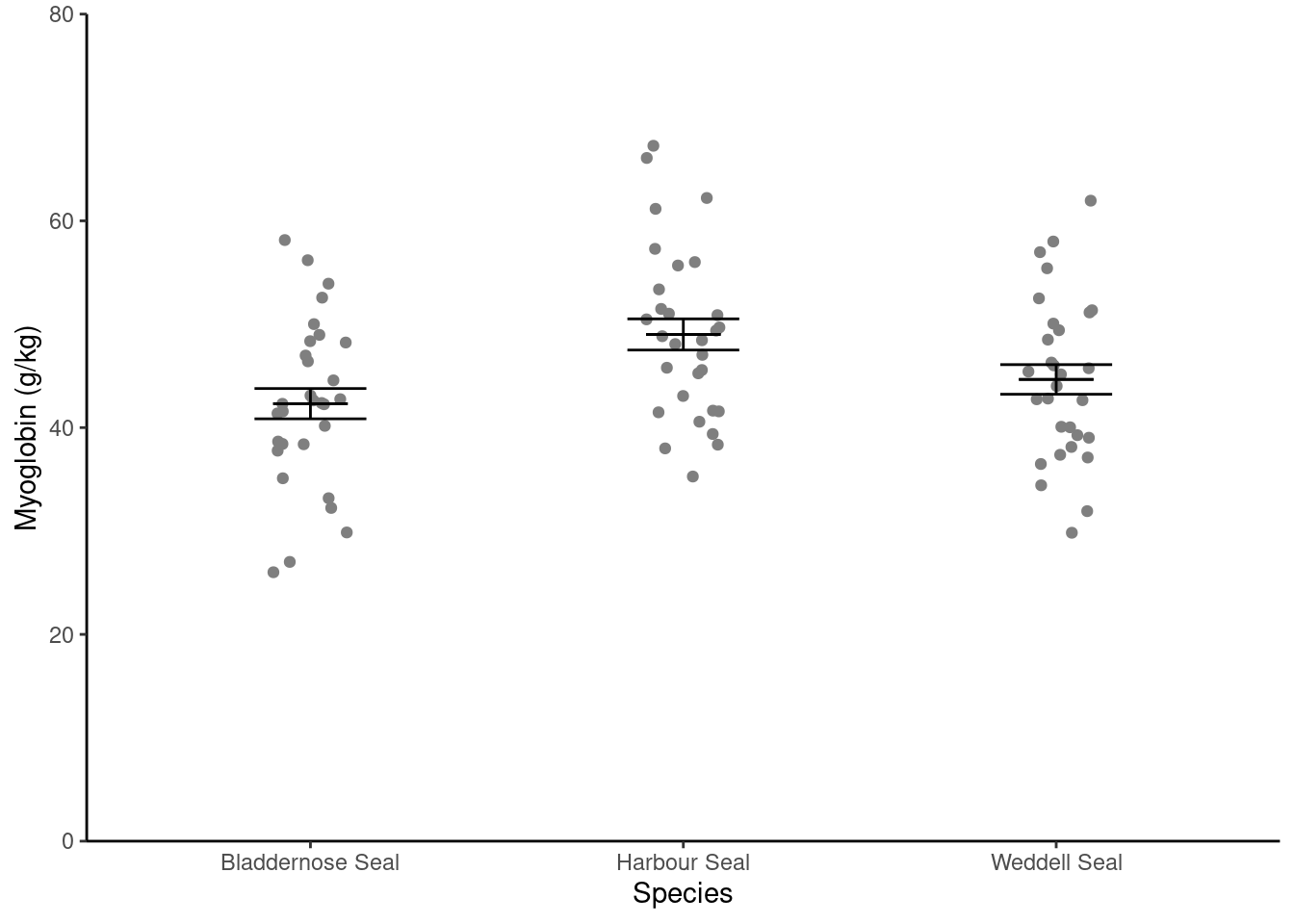
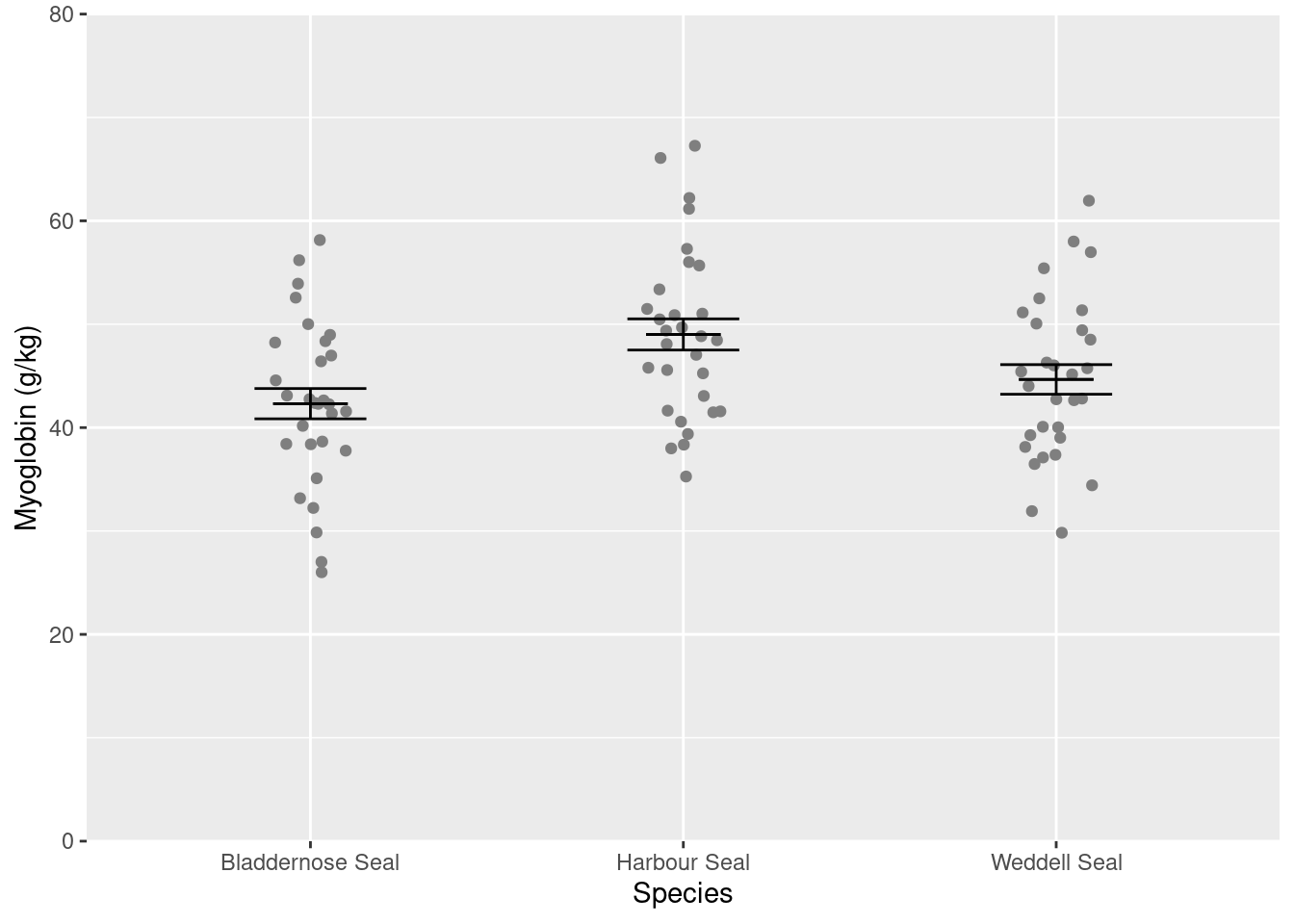
We are going to create a figure like this:
In this figure, we have two dataframes:
- the
sealdataframe which contains the raw data points - the
seal_summarydataframe which contains the means and standard errors.
So far we have plotted just one dataframe on a figure and we have specified the dataframe to be plotted and the columns to plot inside the ggplot() command. For examples:
# YOU DO NOT NEED TO RUN THIS CODE
# It is here to remind you that so far we have specified a single dataframe
# to be plotted and did this by specifying the dataframe to be plotted and
# the columns to plot INSIDE the `ggplot()` command
# For a boxplot ...
ggplot(cats,
aes(x = reorder(coat, mass), y = mass)) +
geom_boxplot(fill = "darkcyan") +
scale_x_discrete(name = "Coat colour") +
scale_y_continuous(name = "Mass (kg)",
expand = c(0, 0),
limits = c(0, 8)) +
theme_classic()Or
# YOU DO NOT NEED TO RUN THIS CODE
# ... and for a histogram
ggplot(fly_bristles_means, aes(x = mean_count)) +
geom_histogram(bins = 10,
colour = "black",
fill = "skyblue") +
scale_x_continuous(name = "Number of bristles",
expand = c(0, 0)) +
scale_y_continuous(name = "Frequency",
expand = c(0, 0),
limits = c(0, 35)) +
theme_classic()Here you will learn that dataframes and aesthetics can be specified within a geom_xxxx rather than within ggplot(). This is very useful if the geom only applies to some of the data you want to plot.
ggplot()
You put the data argument and aes() inside ggplot() if you want all the geoms to use that dataframe and variables. If you want a different dataframe for a geom, put the data argument and aes() inside the geom_xxxx()
I will build the plot up in small steps but you should edit your existingggplot() command as we go.
 Plot the raw data points first:
Plot the raw data points first:
Notice:
geom_point()is the geom to plot pointswe have given the data argument and the aesthetics inside the geom.
the variables given in the aes(),
speciesandmyoglobin, are columns in the dataframe,seal, given in the data argument.
 We can ensure the points do not overlap, by adding some random jitter in the x direction (edit your existing code):
We can ensure the points do not overlap, by adding some random jitter in the x direction (edit your existing code):
ggplot() +
geom_point(data = seal,
aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0))Notice:
position = position_jitter(width = 0.1, height = 0)is inside thegeom_point()parentheses, after theaes()and a comma.We’ve set the vertical jitter to 0 because, in contrast to the categorical x-axis, movement on the y-axis has meaning (the myoglobin levels).
Now to add the errorbars. These go from one standard error below the mean to one standard error above the mean.
 Add a
Add a geom_errorbar() for errorbars (edit your existing code):
ggplot() +
geom_point(data = seal, aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0)) +
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean - se, ymax = mean + se),
width = 0.3) Notice:
geom_errorbar()is the geom to plot error barswe have given the data argument and the aesthetics inside the geom.
the variables given in the aes(),
species,meanandse, are columns in the dataframe,seal_summary, given in the data argument.
We would like to add the mean. You could use geom_point() but I like to use another geom_errorbar() to get a line by setting ymin and ymax to the mean.
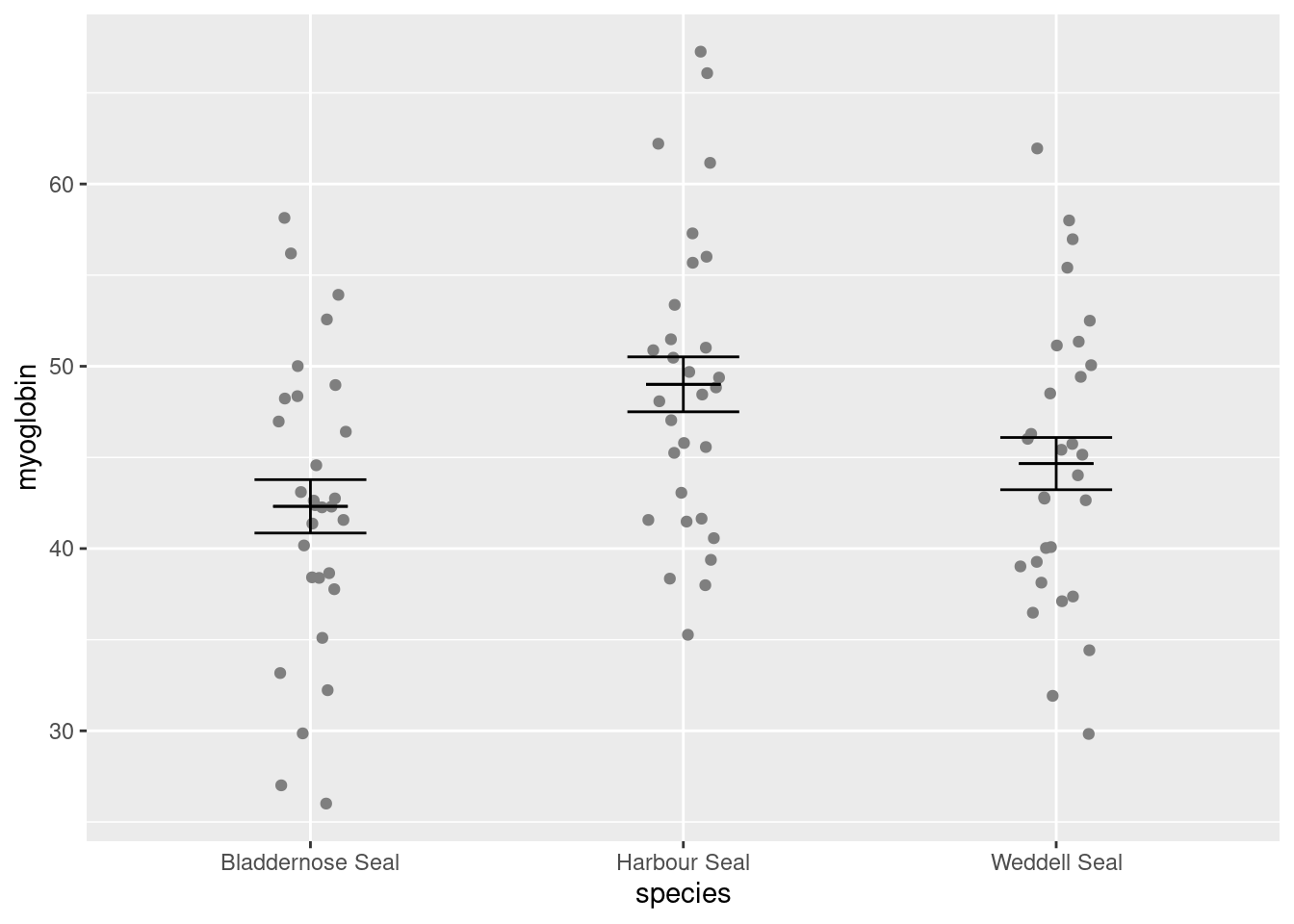
 Add a
Add a geom_errorbar() for the mean (edit your existing code):
ggplot() +
# raw data
geom_point(data = seal, aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0)) +
# error bars
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean - se, ymax = mean + se),
width = 0.3) +
# line for the mean
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean, ymax = mean),
width = 0.2) Alter the axis labels and limits using
Alter the axis labels and limits using scale_y_continuous() and scale_x_discrete() (edit your existing code):
ggplot() +
# raw data
geom_point(data = seal, aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0)) +
# error bars
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean - se, ymax = mean + se),
width = 0.3) +
# line for the mean
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean, ymax = mean),
width = 0.2) +
scale_y_continuous(name = "Myoglobin (g/kg)",
limits = c(0, 80),
expand = c(0, 0)) +
scale_x_discrete(name = "Species")You only need to use name in scale_y_continuous() and scale_x_discrete() to use labels that are different from those in the dataset. Often this is to use proper terminology and captialisation.
 Format the figure in a way that is more suitable for including in a report using
Format the figure in a way that is more suitable for including in a report using theme_classic() (edit your existing code):
ggplot() +
# raw data
geom_point(data = seal, aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0)) +
# error bars
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean - se, ymax = mean + se),
width = 0.3) +
# line for the mean
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean, ymax = mean),
width = 0.2) +
scale_y_continuous(name = "Myoglobin (g/kg)",
limits = c(0, 80),
expand = c(0, 0)) +
scale_x_discrete(name = "Species") +
theme_classic()Writing figures to file
It is a good idea to save figures to files so that you can include them in reports. By scripting figures saving you can ensure your work is reproducible. It means you can regenerate the figures identically if you change the data or exactly match figures in size. Aim to save figures the size they will appear in a report - that way the fonts and line widths will be appropriate. If you need a high resolution figure, you can save it larger than needed then scale it down in the report. Doing that will require you to make changes to font sizes and line widths to make them appropriate when scaled down.
 First make a new folder called
First make a new folder called figures.
 Now edit your ggplot code so that you assign the figure to a variable which you use use in the
Now edit your ggplot code so that you assign the figure to a variable which you use use in the ggsave() command next.
sealfig <- ggplot() +
# raw data
geom_point(data = seal, aes(x = species, y = myoglobin),
position = position_jitter(width = 0.1, height = 0)) +
# error bars
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean - se, ymax = mean + se),
width = 0.3) +
# line for the mean
geom_errorbar(data = seal_summary,
aes(x = species, ymin = mean, ymax = mean),
width = 0.2) +
scale_y_continuous(name = "Myoglobin (g/kg)",
limits = c(0, 80),
expand = c(0, 0)) +
scale_x_discrete(name = "Species") +
theme_classic()The figure won’t be shown in the Plots tab - the output has gone into sealfig rather than to the Plots tab. To make it appear in the Plots tab type sealfig.
 The
The ggsave() command will write a ggplot figure to a file:
ggsave("figures/seal-muscle.png",
plot = sealfig,
device = "png",
width = 4,
height = 3,
units = "in",
dpi = 300)figures/seal-muscle.png is the name of the file, including the relative path.
 Look up
Look up ggsave() in the manual to understand the arguments. You can do this by putting your cursor on the command and pressing F1
Data needs tidying: Pigeons
The data in pigeon.txt are 40 measurements of interorbital width (in mm) for two populations of domestic pigeons measured to the nearest 0.1mm
Import
 Save pigeon.txt to your
Save pigeon.txt to your data-raw folder.
 Read the data into a dataframe called
Read the data into a dataframe called pigeons.
 What variables are there in the
What variables are there in the pigeons dataframe?
Hummmm, these data are not organised like the other data sets we have used. The population is given as the column names and the interorbital distances for one population are given in a different column than those for the other population. The first row has data from two pigeons which have nothing in common, they just happen to be the first individual recorded in each population.
| A | B |
|---|---|
| 12.4 | 12.6 |
| 11.2 | 11.3 |
| 11.6 | 12.1 |
| 12.3 | 12.2 |
| 11.8 | 11.8 |
| 10.7 | 11.5 |
| 11.3 | 11.2 |
| 11.6 | 11.9 |
| 12.3 | 11.2 |
| 10.5 | 12.1 |
| 12.1 | 11.9 |
| 10.4 | 10.7 |
| 10.8 | 11.0 |
| 11.9 | 12.2 |
| 10.9 | 12.6 |
| 10.8 | 11.6 |
| 10.4 | 10.7 |
| 12.0 | 12.4 |
| 11.7 | 11.8 |
| 11.3 | 11.1 |
| 11.5 | 12.9 |
| 11.8 | 11.9 |
| 10.3 | 11.1 |
| 10.3 | 12.2 |
| 11.5 | 11.8 |
| 10.7 | 11.5 |
| 11.3 | 11.2 |
| 11.6 | 11.9 |
| 13.3 | 11.2 |
| 10.7 | 11.1 |
| 12.1 | 11.6 |
| 10.2 | 12.7 |
| 10.8 | 11.0 |
| 11.4 | 12.2 |
| 10.9 | 11.3 |
| 10.3 | 11.6 |
| 10.4 | 12.2 |
| 10.0 | 12.4 |
| 11.2 | 11.3 |
| 11.3 | 11.1 |
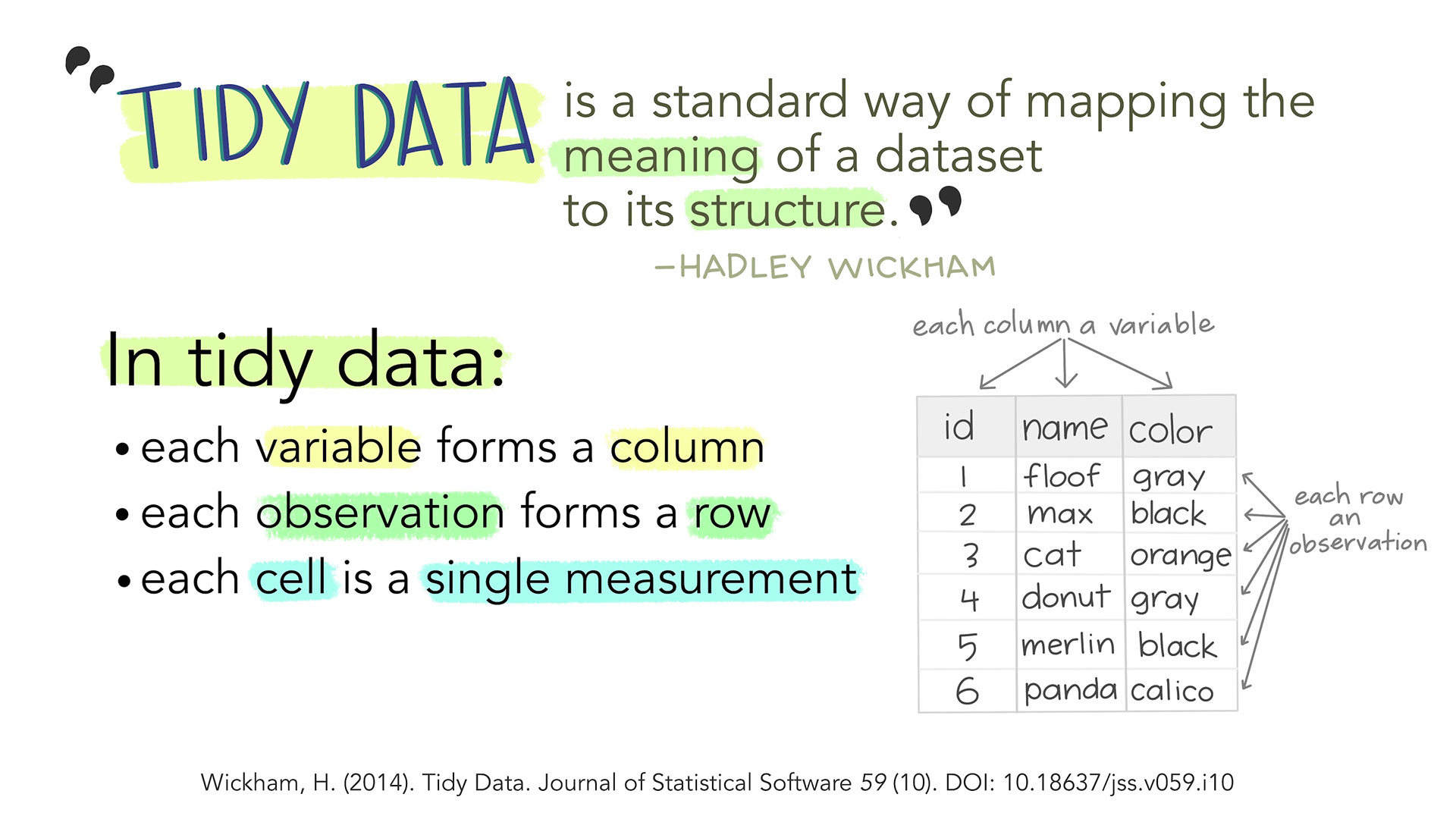
This data is not in ‘tidy’ format (Wickham 2014).
Tidy format has variables in columns and observations in rows. All of the distance measurements should be in one column with a second column giving the population.
| population | distance |
|---|---|
| A | 12.4 |
| B | 12.6 |
| A | 11.2 |
| B | 11.3 |
| A | 11.6 |
| B | 12.1 |
| A | 12.3 |
| B | 12.2 |
| A | 11.8 |
| B | 11.8 |
| A | 10.7 |
| B | 11.5 |
| A | 11.3 |
| B | 11.2 |
| A | 11.6 |
| B | 11.9 |
| A | 12.3 |
| B | 11.2 |
| A | 10.5 |
| B | 12.1 |
| A | 12.1 |
| B | 11.9 |
| A | 10.4 |
| B | 10.7 |
| A | 10.8 |
| B | 11.0 |
| A | 11.9 |
| B | 12.2 |
| A | 10.9 |
| B | 12.6 |
| A | 10.8 |
| B | 11.6 |
| A | 10.4 |
| B | 10.7 |
| A | 12.0 |
| B | 12.4 |
| A | 11.7 |
| B | 11.8 |
| A | 11.3 |
| B | 11.1 |
| A | 11.5 |
| B | 12.9 |
| A | 11.8 |
| B | 11.9 |
| A | 10.3 |
| B | 11.1 |
| A | 10.3 |
| B | 12.2 |
| A | 11.5 |
| B | 11.8 |
| A | 10.7 |
| B | 11.5 |
| A | 11.3 |
| B | 11.2 |
| A | 11.6 |
| B | 11.9 |
| A | 13.3 |
| B | 11.2 |
| A | 10.7 |
| B | 11.1 |
| A | 12.1 |
| B | 11.6 |
| A | 10.2 |
| B | 12.7 |
| A | 10.8 |
| B | 11.0 |
| A | 11.4 |
| B | 12.2 |
| A | 10.9 |
| B | 11.3 |
| A | 10.3 |
| B | 11.6 |
| A | 10.4 |
| B | 12.2 |
| A | 10.0 |
| B | 12.4 |
| A | 11.2 |
| B | 11.3 |
| A | 11.3 |
| B | 11.1 |
Data which is in tidy format is easier to summarise, analyses and plot because the organisation matches the conceptual structure of the data:
- it is more obvious what the variables are because they columns are named with them - in the untidy format, that the measures are distances is not clear, and what A and B are isn’t clear
- it is more obvious that there is no relationship between any of the pigeons except for population
- functions are designed to work with variables in columns
Tidying data
We can put this data in such a format with the pivot_longer() function from the tidyverse:
pivot_longer() collects the values from specified columns (cols) into a single column (values_to) and creates a column to indicate the group (names_to).
 Put the data in tidy format:
Put the data in tidy format:
pigeons <- pivot_longer(data = pigeons,
cols = everything(),
names_to = "population",
values_to = "distance")We have overwritten the original dataframe. If you wanted to keep the original you would need to give a new name on the left side of the assignment <- Note: the actual data in the file are unchanged, only the dataframe in R is changed.
Ulna and height
The datasets we have used up to this point, have had a continuous variable and a categorical variable where it makes sense to summarise the response for each of the different groups in the categorical variable and plot the response on the y-axis. We will now summarise a dataset with two continuous variables. The data in height.txt are the ulna length (cm) and height (m) of 30 people. In this case, it is more appropriate to summarise both of thee variables and to plot them as a scatter plot.
We will use summarise() again but we do not need the group_by() function this time. We will also need to use each of the summary functions, such as mean(), twice, once for each variable.
Import
 Save height.txt to your
Save height.txt to your data-raw folder
 Read the data into a dataframe called
Read the data into a dataframe called ulna_heights.
Summarise
 Create a data frame called
Create a data frame called ulna_heights_summary that contains the sample size and means, standard deviations and standard errors for both variables.
You should get the following numbers:
| n | mean_ulna | std_ulna | se_ulna | mean_height | std_height | se_height |
|---|---|---|---|---|---|---|
| 30 | 24.72 | 4.137332 | 0.75537 | 1.494 | 0.2404823 | 0.0439059 |
Visualise
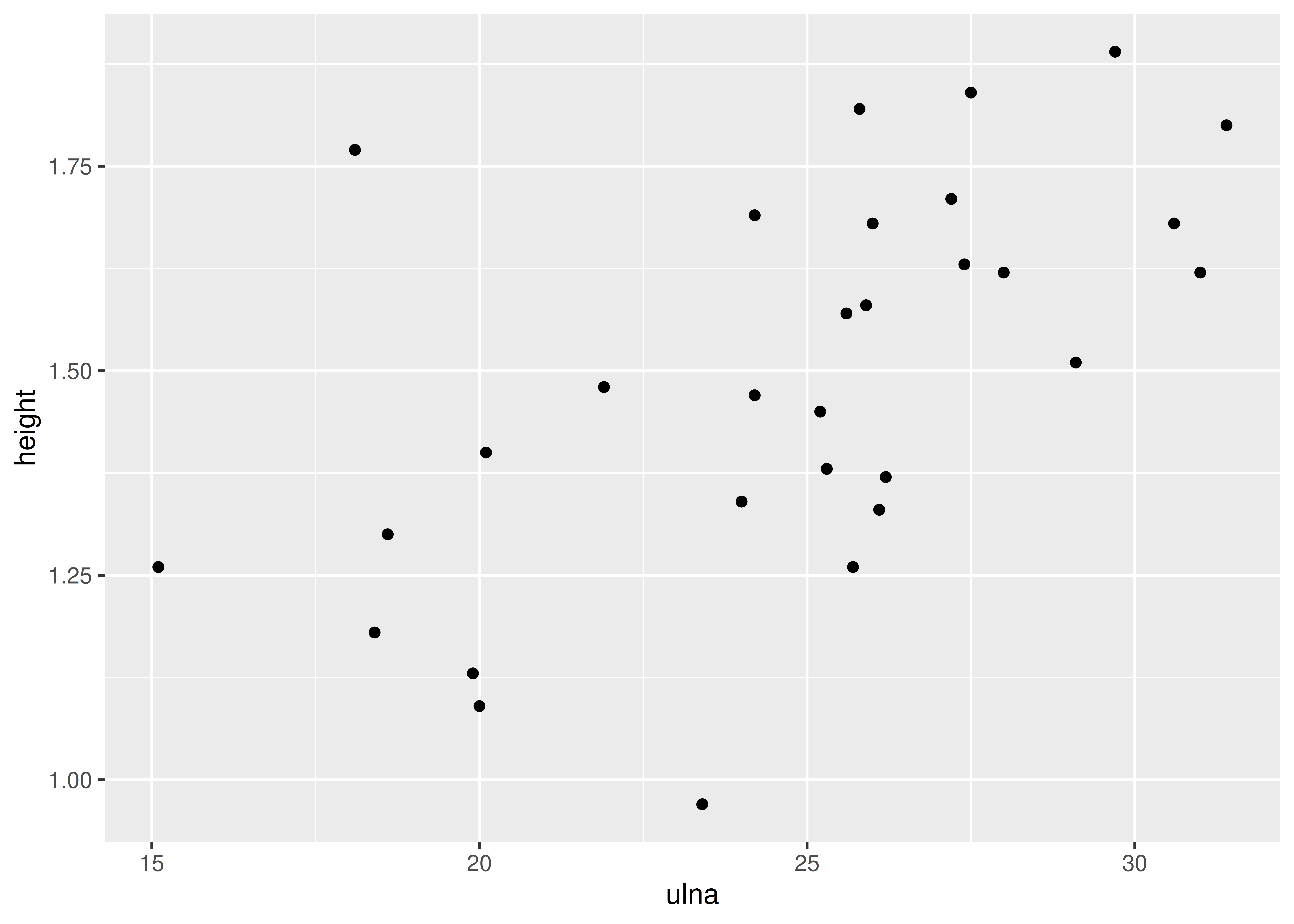
To plot make a scatter plot we need to use geom_point() again but without any scatter. In this case, it does not really matter which variable is on the x-axis and which is on the y-axis.
 Make a simple scatter plot:
Make a simple scatter plot:
If you have time, you may want to format the figure more appropriately.
Look after future you!
Future you is going to summarise and plot data from other modules. You can make this much easier by documenting what you have done now. Go through your script (summarise-plot-data-with-several-vars.R) and remove code that you do not need. Add comments to the code you do need to explain what it does.
You’re finished!
🥳 Well Done! 🎉
Independent study following the workshop
The Code file
This contains all the code needed in the workshop even where it is not visible on the webpage.
The workshop.qmd file is the file I use to compile the practical. Qmd stands for Quarto markdown. It allows code and ordinary text to be interweaved to produce well-formatted reports including webpages. View the source code for this workshop using the </> Code button at the top of the page. Coding and thinking answers are marked with #---CODING ANSWER--- and #---THINKING ANSWER---
Pages made with R (R Core Team 2024), Quarto (Allaire et al. 2022), knitr (Xie 2024, 2015, 2014), kableExtra (Zhu 2024)
References
Footnotes
“Create a dataframe called
seal_summary” means assign the output of thegroup_by()andsummarise()code to something calledseal_summary.↩︎