| species | mean | std | n | se |
|---|---|---|---|---|
| Bladdernose Seal | 42.31600 | 8.020634 | 30 | 1.464361 |
| Harbour Seal | 49.01033 | 8.252004 | 30 | 1.506603 |
| Weddell Seal | 44.66033 | 7.849816 | 30 | 1.433174 |
Workshop
One-way ANOVA and Kruskal-Wallis
Introduction
Session overview
In this session you will get practice in choosing between, performing, and presenting the results of, one-way ANOVA and Kruskal-Wallis in R.
Philosophy
Workshops are not a test. It is expected that you often don’t know how to start, make a lot of mistakes and need help. It is expected that you are familiar with independent study content before the workshop. However, you need not remember or understand every detail as the workshop should build and consolidate your understanding.
Tips
- don’t worry about making mistakes
- don’t let what you can not do interfere with what you can do
- discussing code with your neighbours will help
- look things up in the independent study material
- look things up in your own code from earlier
- there are no stupid questions
These four symbols are used at the beginning of each instruction so you know where to carry out the instruction.
 Something you need to do on your computer. It may be opening programs or documents or locating a file.
Something you need to do on your computer. It may be opening programs or documents or locating a file.
 Something you should do in RStudio. It will often be typing a command or using the menus but might also be creating folders, locating or moving files.
Something you should do in RStudio. It will often be typing a command or using the menus but might also be creating folders, locating or moving files.
 Something you should do in your browser on the internet. It may be searching for information, going to the VLE or downloading a file.
Something you should do in your browser on the internet. It may be searching for information, going to the VLE or downloading a file.
 A question for you to think about and answer. Record your answers in your script for future reference.
A question for you to think about and answer. Record your answers in your script for future reference.
Getting started
 Start RStudio from the Start menu.
Start RStudio from the Start menu.
 Make an RStudio project for this workshop by clicking on the drop-down menu on top right where it says
Make an RStudio project for this workshop by clicking on the drop-down menu on top right where it says Project: (None) and choosing New Project, then New Directory, then New Project. Navigate to the data-analysis-in-r-2 folder and name the RStudio Project week-4.
 Make new folders called
Make new folders called data-raw and figures. You can do this on the Files Pane by clicking New Folder and typing into the box that appears.
 Make a new script then save it with a name like
Make a new script then save it with a name like one-way-anova-and-kw.R to carry out the rest of the work.
 Add a comment to the script:
Add a comment to the script: # One-way ANOVA and Kruskal-Wallis and load the tidyverse (Wickham et al. 2019) package
Exercises
Myoglobin in seal muscle
The myoglobin concentration of skeletal muscle of three species of seal in grams per kilogram of muscle was determined and the data are given in seal.csv. We want to know if there is a difference between species. Each row represents an individual seal. The first column gives the myoglobin concentration and the second column indicates species.
 Save a copy of the data file seal.csv to
Save a copy of the data file seal.csv to data-raw
 Read in the data and check the structure. I used the name
Read in the data and check the structure. I used the name seal for the dataframe/tibble.
 What kind of variables do you have?
What kind of variables do you have?
Exploring
 Do a quick plot of the data. You may need to refer to a previous workshop
Do a quick plot of the data. You may need to refer to a previous workshop
Summarising the data
Do you remember Look after future you!
 If you followed that tip you’ll be able to open that script and whizz through summarising,testing and plotting.
If you followed that tip you’ll be able to open that script and whizz through summarising,testing and plotting.
 Create a data frame called
Create a data frame called seal_summary that contains the means, standard deviations, sample sizes and standard errors for each species.
You should get the following numbers:
Applying, interpreting and reporting
We can now carry out a one-way ANOVA using the same lm() function we used for two-sample tests.
 Carry out an ANOVA and examine the results with:
Carry out an ANOVA and examine the results with:
Call:
lm(formula = myoglobin ~ species, data = seal)
Residuals:
Min 1Q Median 3Q Max
-16.306 -5.578 -0.036 5.240 18.250
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 42.316 1.468 28.819 < 2e-16 ***
speciesHarbour Seal 6.694 2.077 3.224 0.00178 **
speciesWeddell Seal 2.344 2.077 1.129 0.26202
---
Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
Residual standard error: 8.043 on 87 degrees of freedom
Multiple R-squared: 0.1096, Adjusted R-squared: 0.08908
F-statistic: 5.352 on 2 and 87 DF, p-value: 0.006427Remember: the tilde (~) means test the values in myoglobin when grouped by the values in species. Or explain myoglobin with species
 What do you conclude so far from the test? Write your conclusion in a form suitable for a report.
What do you conclude so far from the test? Write your conclusion in a form suitable for a report.
 Can you relate the values under
Can you relate the values under Estimate to the means?
The ANOVA is significant but this only tells us that species matters, meaning at least two of the means differ. To find out which means differ, we need a post-hoc test. A post-hoc (“after this”) test is done after a significant ANOVA test. There are several possible post-hoc tests and we will be using Tukey’s HSD (honestly significant difference) test (Tukey 1949) implemented in the emmeans (Lenth 2024) package.
 Load the package
Load the package
 Carry out the post-hoc test
Carry out the post-hoc test
contrast estimate SE df t.ratio p.value
Bladdernose Seal - Harbour Seal -6.69 2.08 87 -3.224 0.0050
Bladdernose Seal - Weddell Seal -2.34 2.08 87 -1.129 0.4990
Harbour Seal - Weddell Seal 4.35 2.08 87 2.095 0.0968
P value adjustment: tukey method for comparing a family of 3 estimates Each row is a comparison between the two means in the ‘contrast’ column. The ‘estimate’ column is the difference between those means and the ‘p.value’ indicates whether that difference is significant.
 What do you conclude from the test?
What do you conclude from the test?
Check assumptions
The assumptions of the general linear model are that the residuals – the difference between predicted value (i.e., the group mean) and observed values - are normally distributed and have homogeneous variance. To check these we can examine the mod$residuals variable. You may want to refer to Checking assumptions in the “Single regression” workshop.
 Plot the model residuals against the fitted values.
Plot the model residuals against the fitted values.
 What to you conclude?
What to you conclude?
To examine normality of the model residuals we can plot them as a histogram and do a normality test on them.
 Plot a histogram of the residuals.
Plot a histogram of the residuals.
 Use the
Use the shapiro.test() to test the normality of the model residuals
 What to you conclude?
What to you conclude?
Illustrating
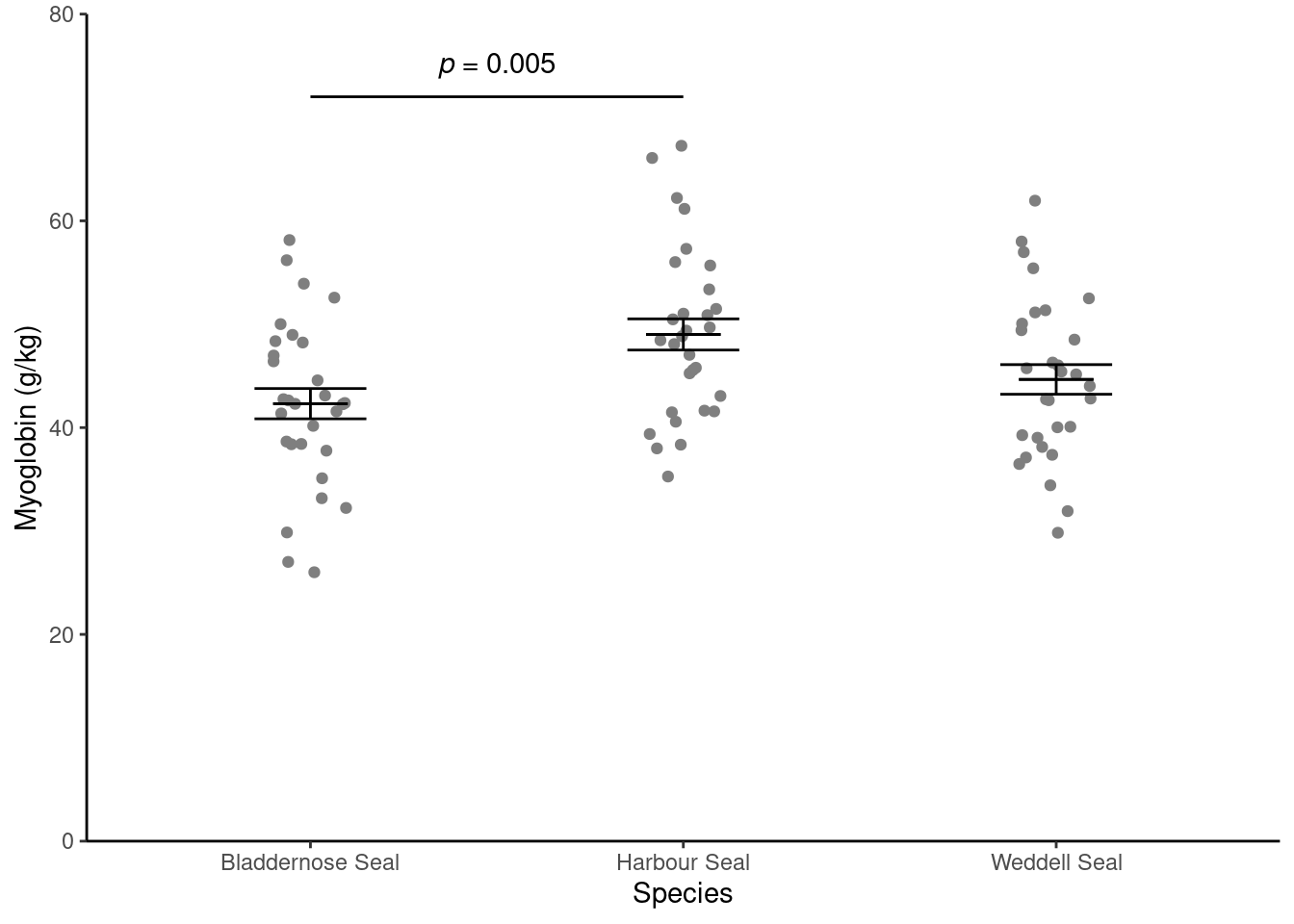
 Create a figure like the one below. You may need to refer to:
Create a figure like the one below. You may need to refer to:
Visualise from the “Summarising data with several variables” workshop (Rand 2023)
The week 3 workshop (Rand 2023) for annotating. Remember, the lines go between two means that differ significantly.
We will again use both our seal and seal_summary dataframes.
 Create the plot
Create the plot
 Save your figure to your figures folder.
Save your figure to your figures folder.
Leafminers on Birch
Larvae of the Ambermarked birch leafminer, Profenusa thomsoni, feed on the interior leaf tissues of Birch (Betula) species. They do not normally kill the tree but can weaken it making it susceptible to attack from other species. Researchers are interested in whether there is a difference in the rates at which white, grey and yellow birch are attacked. They introduce adult female P.thomsoni to a green house containing 30 young trees (ten of each type) and later count the egg laying events on each tree. The data are in leaf.txt.
Exploring
 Read in the data and check the structure. I used the name
Read in the data and check the structure. I used the name leaf for the dataframe/tibble.
 What kind of variables do we have?
What kind of variables do we have?
 Do a quick plot of the data.
Do a quick plot of the data.
 Using your common sense, do these data look normally distributed?
Using your common sense, do these data look normally distributed?
 Why is a Kruskal-Wallis appropriate in this case?
Why is a Kruskal-Wallis appropriate in this case?
 Calculate the medians, means and sample sizes.
Calculate the medians, means and sample sizes.
Applying, interpreting and reporting
 Carry out a Kruskal-Wallis:
Carry out a Kruskal-Wallis:
kruskal.test(data = leaf, eggs ~ birch)
Kruskal-Wallis rank sum test
data: eggs by birch
Kruskal-Wallis chi-squared = 6.3393, df = 2, p-value = 0.04202 What do you conclude from the test?
What do you conclude from the test?
A significant Kruskal-Wallis tells us at least two of the groups differ but where do the differences lie? The Dunn test is a post-hoc multiple comparison test for a significant Kruskal-Wallis. It is available in the package FSA
 Load the package using:
Load the package using:
 Run the post-hoc test with:
Run the post-hoc test with:
dunnTest(data = leaf, eggs ~ birch) Comparison Z P.unadj P.adj
1 Grey - White 1.296845 0.19468465 0.38936930
2 Grey - Yellow -1.220560 0.22225279 0.22225279
3 White - Yellow -2.517404 0.01182231 0.03546692The P.adj column gives p-value for the comparison listed in the first column. Z is the test statistic.
 What do you conclude from the test?
What do you conclude from the test?
 Write up the result is a form suitable for a report.
Write up the result is a form suitable for a report.
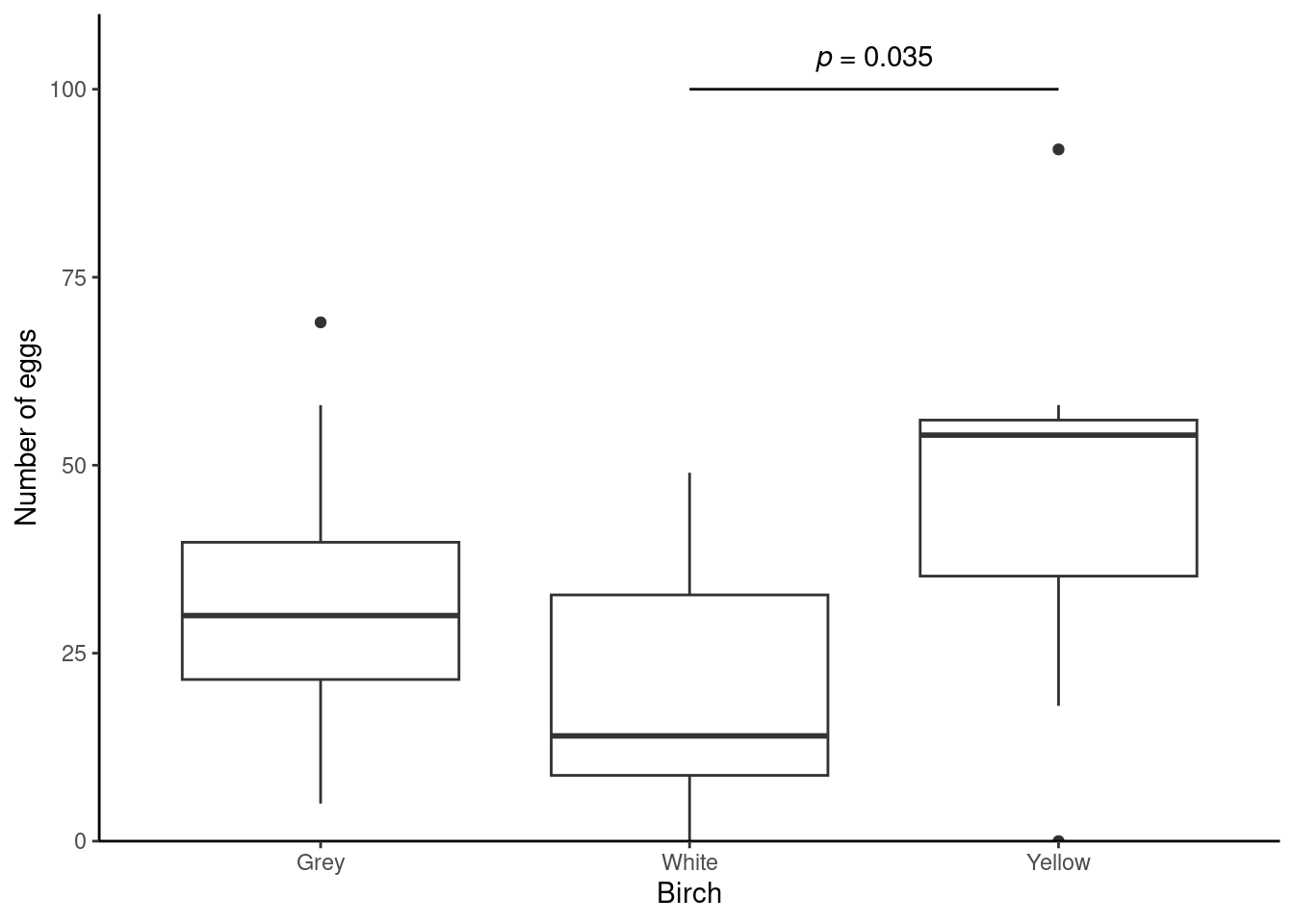
Illustrating
 A box plot is an appropriate choice for illustrating a Kruskal-Wallis. Can you produce a figure like this?
A box plot is an appropriate choice for illustrating a Kruskal-Wallis. Can you produce a figure like this?
You’re finished!
🥳 Well Done! 🎉
Independent study following the workshop
The Code file
All the answers (coding and thinking) are in the source code for the workshop. You can view it using the </> Code button at the top of the page. Coding and thinking answers are marked with #---CODING ANSWER--- and #---THINKING ANSWER---
Pages made with R (R Core Team 2024), Quarto (Allaire et al. 2022), knitr (Xie 2024, 2015, 2014), kableExtra (Zhu 2024)